B-T-E’s Patented CO2 Conversion Technology to Syngas to Energy
An economical commercial process is needed to provide an incentive for the utility industries to engender win-win support for Governmental regulations on Carbon dioxide (CO2) emissions. Current approach to mitigating CO2 emissions is carbon capture and sequestration (CCS) which involves CO2 capture followed by CO2 sequestration involving costly CO2 compression, transportation, underground storage and/or used for Crude Oil recovery from reservoirs.
Alternative proprietary patented processes, both non-catalytic process as well as a catalytic process, have been developed for mitigating CO2 emissions which solidifies the aims of both parties, i.e., industry and government. Both processes, the “non-catalytic” process and the “catalytic” process by B-T-E were developed for mitigating CO2 emissions from industrial plants by conversion to fuels. B-T-E’s patented CO2 conversion processes convert CO2 into Syngas (CO & H2) with further conversion to fuels such as Gasoline, Diesel, Jet Fuel, Hydrogen, Methanol, and/or Ethanol with established mature technologies. The patented technology for the conversion of CO2 to Syngas was developed by B-T-E and has seven (7) U.S. patents, one (1) Japanese patent, European Patent (EP) patents, one (1) India patent, one (1) Brazil patent, and other patents pending. B-T-E’s proprietary “catalytic” technology is referred to as the patented SMR+® process.
B-T-E has spent about 10 years in the development of the patented CO2 conversion technology to syngas (CO & H2) and potential commercial applications. Originally, thermodynamic computations by Dr. Gary Young of Bio-Thermal-Energy Inc. determined that CO2 could be converted into syngas with a carbonaceous material and steam at proper process conditions using gasification.B-T-E has continued development of this novel proprietary technology which currently has shown to be a game changer for the energy industry and will be shown as the process whether it be the catalytic or non-catalytic. B-T-E, Inc. owns and holds the rights to the Patented B-T-E CO2 Conversion Technology.
Some applications of using B-T-E’s Patented CO2 Conversion Technology are as follows to mention a few:
- Carbon dioxide (CO2) from Stack gas emissions of Coal-fired Power Plants converted to Gasoline,
- Carbon dioxide (CO2) emissions from fermenters of Corn-Ethanol Plants converted into Ethanol without using additional Corn, and
- Landfill Gas – LFG (Carbon dioxide- CO2 & Methane– CH4) from Landfills converted to Ethanol.
B-T-E’s CO2 Conversion Technology as applied over-all whether using the Catalytic or Non-catalytic approach for the production of energy:
As an introduction to B-T-E’s Patented CO2 Conversion Technology to Syngas (CO & H2), the following chemistry representation is where the Carbon dioxide (CO2) is from an industrial source:
CO2 + Carbonaceous material + H2O → Gasifier or Reformer → Syngas (CO & H2)
whereby:
- if the Carbonaceous material is a gas, solid and/or liquid, the process is “non-catalytic” and a Gasifier can be used; biomass solids, Coal such as lignite, lignin, Municipal Solid Waste (MSW), Corn stover, switch grass, Landfill gas, etc.,
- if the Carbonaceous material is a gas, such as Natural gas (CH4), and/or some liquids, the process is “catalytic” and a Reformer is used with conventional Reformer and catalysts.
Further processing of the Syngas (CO & H2) is done using a proven well-established commercial process to produce fuels such as Gasoline, Diesel, Jet Fuel, Methanol, Ethanol, etc., as illustrated:
Syngas (CO & H2) → Fuels (Gasoline, Diesel, Jet Fuel, Methanol, Ethanol, etc.)
The over-all process is illustrated as:
CO2 + Carbonaceous Material + H2O → Syngas (CO & H2) → Fuels
and with B-T-E’s patented SMR+® (Steam-Methane Reforming-plus) technology, U.S. Patent 9,212,059, as applied to making Gasoline, for example:
CO2 + CH4 (Natural gas) + H2O → Reformer [Syngas (CO & H2)] → Fischer-Tropsch (Gasoline)
The over-all process including B-T-E’s CO2 Conversion Technology with Syngas further converted to Fuels/Energy is illustrated below:

Examples for the application of B-T-E’s patented CO2 conversion are presented below for the products gasoline and Ethanol.
- Example A is Coal-fired CO2 emissions to produce Gasoline using B-T-E’s CO2 Catalytic technology.
- Example B is Coal-fired CO2 emissions to produce Gasoline using B-T-E’s CO2 Non-Catalytic technology.
- Example C is CO2 emissions from a Corn-Ethanol plant to produce additional Ethanol without using more Corn.
- Example D is Landfill Gas (CO2 & CH4) emissions from a Landfill to produce Ethanol.
- Example E is conversion of Landfill Gas (LFG) into Ethanol.
Example A: B-T-E’s CO2 Opportunity, Patented "Catalytic" SMR+® Technology; Converting CO2 Emissions from Coal-fired Power Plants to Gasoline
The Process
Figure below provides a pictorial representation of the B-T-E technology as used for the conversion of CO2 emissions from a representative coal-fired power plant (790 MW) to gasoline, with an estimated production of 137,200 barrels/day using B-T-E’s SMR+® “Catalytic” Technology.
 Carbon dioxide Conversion to Gasoline using B-T-E’s SMR+® “catalytic” Technology
Carbon dioxide Conversion to Gasoline using B-T-E’s SMR+® “catalytic” Technology Step 1 – Capturing Emissions
Coal-fired stack gas emissions are sent to a carbon dioxide capture plant to remove CO2 from the stack gas. The stack gas is comprised mainly of Nitrogen (about 70% vol.), water, CO2 (about 20%), and impurities of SO2, NOx, and mercury. CO2 capture system can recover up to about 90% of the CO2 from the stack gas such as by Shell Oil Company CO2 capture system.
Step 2 – Conversion to Syngas
CO2 is then converted to Syngas (mostly CO & H2) with B-T-E’s proprietary technology in a CO2-to-Syngas process plant. Note, B-T-E’s novel technology has been proven experimentally on a gasification pilot plant with a capacity of 12.5 TPD (tons per day). Pilot plant tests have experimentally verified a reduction of CO2 of about 70 percent, with significant improvements anticipated with further optimization.
This second step involves B-T-E’s patented SMR+® catalytic technology. Carbon dioxide (CO2), natural gas (methane, CH4), and steam are fed to a Reformer to produce Syngas as illustrated below:
CO2+ Methane (CH4) + Steam (H2O) → Syngas (CO & H2)
Note, this step uses the typical Steam-Methane Reformer process but B-T-E’s SMR+® process utilizes an independent external supply of Carbon dioxide (CO2), U.S. Patent 9,212,059.
Step 3 – Conversion to Gasoline
Syngas is then fed to a syngas-to-gasoline plant for the conversion of syngas to gasoline, such as by using ExxonMobil’s GTL (gas to liquids) process, as illustrated:
Syngas (CO & H2) → Gasoline
The Economics:
With B-T-E’s patented SMR+® catalytic process coupled with CO2 Capture process and GTL process to Gasoline, the over-all process to convert CO2 emissions from a coal-fired power plant into Gasoline becomes:
CO2 + Methane (CH4) + Steam (H2O) → Syngas (CO & H2) → Gasoline
Figure below illustrates the overall economics of using carbon dioxide emissions from a representative 790-MW coal-fired power plant to produce gasoline, using B-T-E’s patented SMR+® "Catalytic" proprietary technology, in terms of gasoline production costs as a function of the wholesale natural gas price and retail industrial rate for electricity.
NOTE: The cost of Post Combustion Carbon Capture (CO2 capture) is included in the production cost of Gasoline.

A 790 MW Coal-fired Power Plant with stack gas emissions of about 775 tons/hr can produce about 137,200 barrels/day of Gasoline at a production cost of $0.58/gallon gasoline with Natural gas at $2.00/MMBtu and electricity at $0.0500/kWh using B-T-E’s patented SMR+® "Catalytic" Technology. Thus, CO2-Gasoline has a production cost of $0.58/gallon gasoline using SMR+® "Catalytic" technology. In comparison, the production cost of Gasoline from the traditional Crude Oil-to-Gasoline refinery would be about $1.26/gallon gasoline with Crude Oil selling between $30-$60/barrel. Thus, it indicates that with the unique and patented SMR+® "catalytic" process, CO2-to-Gasoline process is competitive with the Crude-to-Gasoline process. Note, about 6.46 lbs of CO2 Stack Gas emissions from a Coal-fired Power Plant are used to produce a gallon of Gasoline.
Even with Natural Gas cost at $4.00/MM BTU, the production cost is about $0.98/gallon of gasoline.
Also, Tailgas from the CO2-Gasoline Plant produces 708 MW of Utility Power as Net Export to the GRID.
As a reminder, Production Cost of Gasoline “includes” the cost of Post Combustion Carbon Capture (CO2 capture). The Coal-fired Power Plant just supplies the Stack Gas to the CO2-Gasoline Plant.
B-T-E's proprietary process technology can be used to produce fuels such as Gasoline, Diesel, and/or Jet Fuel.
The conversion of Carbon dioxide (CO2) from the stack gas emissions of a Coal-fired Power Plant to Gasoline using B-T-E Patented SMR+® "Catalytic"Conversion Technology was published in Hydrocarbon Processing, i.e., “Mitigate CO2 emissions from industrial plants by conversion to fuels,” HP Special Focus | Clean Fuels, G. C. Young, Bio-Thermal-Energy, Inc., Cedar Rapids, Iowa, Hydrocarbon Processing | FEBRUARY 2017.
Example B: B-T-E’s CO2 Opportunity, Patented CO2 Conversion "Non-catalytic" Technology; Converting CO2 Emissions from Coal-fired Power Plants to Gasoline
The Process
Figure below provides a pictorial representation of the B-T-E "Non-catalytic" technology as used for the conversion of CO2 emissions from a representative coal-fired power plant (790 MW) to gasoline, with an estimated production of 137,200 barrels/day.
 Carbon dioxide Conversion to Gasoline using B-T-E’s Gasification “non-catalytic” Process Technology
Carbon dioxide Conversion to Gasoline using B-T-E’s Gasification “non-catalytic” Process Technology
Step 1 – Capturing Emissions
Coal-fired stack gas emissions are sent to a carbon dioxide capture plant to remove CO2 from the stack gas. The stack gas is comprised mainly of Nitrogen (about 70% vol.), water, CO2 (about 20%), and impurities of SO2, NOx, and mercury. CO2 capture system can recover up to about 90% of the CO2 from the stack gas such as by Shell Oil Company CO2 capture system.
Step 2 – Conversion to Syngas
CO2 is then converted to Syngas (mostly CO & H2) with B-T-E’s proprietary technology in a CO2-to-Syngas process plant. Note, B-T-E’s novel technology has been proven experimentally on a gasification pilot plant with a capacity of 12.5 TPD (tons per day). Pilot plant tests have experimentally verified a reduction of CO2 of about 70 percent, with significant improvements anticipated with further optimization.
This second step involves B-T-E’s patented gasification non-catalytic technology. Carbon dioxide (CO2), natural gas (methane, CH4), and steam are fed to a Gasifier to produce Syngas as illustrated below:
CO2 + Methane (CH4) + Steam (H2O) → Syngas (CO & H2)
Step 3 – Conversion to Gasoline
Syngas is then fed to a syngas-to-gasoline plant for the conversion of syngas to gasoline, such as by using ExxonMobil’s GTL (gas to liquids) process, as illustrated:
Syngas (CO & H2) → Gasoline
The Economics:
With B-T-E’s patented non-catalytic process technology coupled with CO2 Capture process and GTL process to Gasoline, the over-all process to convert CO2 emissions from a coal-fired power plant into Gasoline becomes:
CO2 + Methane (CH4) + Steam (H2O) → Syngas (CO & H2) → Gasoline
Figure below illustrates the overall economics of using carbon dioxide emissions from a representative 790-MW coal-fired power plant to produce gasoline, using B-T-E’s patented “Non-catalytic” gasification process technology, in terms of gasoline production costs as a function of the wholesale natural gas price and retail industrial rate for electricity.
NOTE: The cost of Post Combustion Carbon Capture (CO2 capture) is included in the production cost of Gasoline.

A 790 MW Coal-fired Power Plant with stack gas emissions of about 775 tons/hr can produce about 137,200 barrels/day of Gasoline at a production cost of $0.97/gallon gasoline with Natural gas at $2.00/MMBtu and electricity at $0.0550/kWh using B-T-E’s Patented "Non-Catalytic" Technology. Thus, CO2-Gasoline has a production cost of $0.97/gallon gasoline using B-T-E’s patented non-catalytic gasification process technology. In comparison, the production cost of Gasoline from the traditional Crude Oil-to-Gasoline refinery would be about $1.26/gallon gasoline with Crude Oil selling between $30-$60/barrel. Thus, it indicates that with the unique and patented B-T-E’s "non-catalytic" process, CO2-to-Gasoline process is competitive with the Crude-to-Gasoline process. Note, about 6.46 lbs of CO2 Stack Gas emissions from a Coal-fired Power Plant are used to produce a gallon of Gasoline.
Even with Natural Gas cost at $4.00/MM BTU, the production cost is about $1.26/gallon of gasoline.
B-T-E's proprietary process technology can be used to produce fuels such as Gasoline, Diesel, and/or Jet Fuel.
Example C: B-T-E’s CO2 Opportunity, Patented CO2 Conversion Technology; Converting CO2 Emissions from Corn-Ethanol Plants to Additional Ethanol
Before discussing details, let us review the over-all application of B-T-E’s patented SMR+® "catalytic" process technology for the production of Ethanol using the Carbon dioxide (CO2) emissions from the fermenters of a Corn-Ethanol plant. An illustration below shows a Corn-Ethanol plant with a production capacity of 100 MM GPY of Ethanol which produces byproduct CO2 from the fermenters of 37.75 tons/hour CO2, (34.25 tonnes/hour CO2 or 300,000 tonnes/year CO2). The CO2-Ethanol plant can produce another 208.5 MM GPY Ethanol using B-T-E’s patented CO2 Conversion Technology with the byproduct CO2 emissions from the Corn-Ethanol plant.

Corn-Ethanol Plant and a CO2-Ethanol Plant Facility
The facility with a Corn-Ethanol plant and a CO2-Ethanol plant could be known as a Hybrid Ethanol Facility. Corn-Ethanol plant with a capacity for production of 100 MM GPY Ethanol has CO2 emissions of 37.75 tons/hour or (34.25 tonnes/hour CO2 or 300,000 tonnes/year CO2). The CO2-Ethanol plant, using B-T-E’s patented CO2 Conversion Technology, will produce 208.5 MM GPY of Ethanol with a production cost of $0.76/gallon Ethanol and with a Total Investment Capital (TIC) of $583 MM or $2.80 TIC/gallon Ethanol produced. Further details are presented below.
Application of B-T-E’s CO2 Conversion technology to the CO2 emissions from a Corn-Ethanol plant with capacity of 100 MM GPY Ethanol and CO2 emissions of 37.75 tons/hour is as follows.
- CO2 emissions from the Corn-Ethanol plant with capacity of 100 MM GPY ethanol: CO2 rate is 37.75 tons/hour, i.e. (34.25 tonnes/hour CO2 or 300,000 tonnes/year CO2). CO2 effluent composition from the fermentors is 98.0% CO2 with a small amount of water vapor and VOC.
- CO2 is converted into Syngas (CO & H2) with B-T-E’s patented SMR+® "Catalytic" Technology as:
CO2 + CH4 + H2O → SMR+® Reformer → Syngas (CO & H2)
The CO2 conversion to Syngas using a carbonaceous material (such as CH4, for example) and Steam (H2O) was proven experimentally in a 12.5 TPD pilot plant of Westinghouse in Pennsylvania.
SMR+® was substantiated by Technip Stone & Webster Process Technology, Inc. in a Feasibility Study for B-T-E, Inc. in 2014. SMR+® catalytic process conditions were: 1600 oF and 35 PSIA. Steam-Methane reforming, SMR, is a well-established mature technology that has been optimized for productivity and efficiency over many hears of industrial application.
The SMR+® process uses heat, pressure, steam, and catalysts to break methane and CO2 into CO and H2(Syngas) for further processing.
- Syngas (CO & H2) is further fermented to Ethanol
Syngas (CO + H2) → fermentation → Ethanol
Syngas to Ethanol was accomplished by biological fermentation of syngas to Ethanol per an available commercial process. With clean gaseous feedstocks, (CO2, CH4, and H2O) to the Reformer, the Syngas produced is expected to be of relatively clean quality when compared to processes using biomass feedstocks. Other biological fermentation processes are available.
Economics of CO2-Ethanol Production Using B-T-E’s Patented CO2 Conversion Technology
As mentioned previously, Corn-Ethanol plant with a capacity for production of 100 MM GPY Ethanol has a CO2 emissions of 37.75 tons/hour or (34.25 tonnes/hour CO2 or 300,000 tonnes/year CO2). The CO2-Ethanol plant, using B-T-E’s patented CO2 Conversion Technology, will produce 208.5 MM GPY of Ethanol with a production cost of $0.76/gallon Ethanol and with a Total Investment Capital (TIC) of $583 MM or $2.80 TIC/gallon Ethanol produced.
Note: SMR+® is a U.S. trademark of B-T-E, Inc.
Example D: B-T-E’s CO2 Opportunity, Patented CO2 Conversion Technology; Converting Landfill Gas (LFG) (CO2 & CH4 emissions from a Landfill to Ethanol
Bio-Thermal-Energy, Inc. (B-T-E, Inc.) has developed a Patented CO2 Conversion Technology which can be used to convert Landfill Gas (CO2 & CH4) into Syngas (CO & H2) which is further fermented, using a well-established commercial process, to Ethanol. The process is simply illustrated as:
Landfill Gas (CO2 & CH4)→Reformer→Syngas (CO & H2)→Fermentation→Ethanol
Plus MW of Utility Power for Net Export to the GRID.
With a site-specific application, Bio-Thermal-Energy, Inc. desires to conduct a feasibility study for Cedar Rapids Linn County Solid Waste Agency, Site #2, Landfill Gas to Ethanol, i.e., LFG-Ethanol plant at Site-2. LFG-Ethanol plant capacity/size is based upon Landfill Gas rates from a recent study, September 13, 2018, by SCS Engineers for CRLCSWA Site 2 Landfill, Projected Landfill Gas recovery rates, File NO. 27217406.00.
The Bio-Thermal-Energy Process for converting Landfill Gas (LFG) to Ethanol is further shown as:
Landfill Gas to Ethanol:
- Landfill Gas is fed to the Reformer or Gasifier.
- Reformer or Gasifier converts the Landfill Gas to Syngas (CO & H2) using B-T-E’s Patented CO2 Conversion Technology.
- Syngas is fermented to Ethanol using a well-established commercial process.
Basic Process Chemistry: LFG to Syngas followed by “fermentation” to Ethanol
LFG (CO2 + CH4) → Syngas (CO & H2) → “fermentation to Ethanol”
C + CO2 → 2 CO [Boudouard Reaction]
C + H2O → CO + H2
CO + H2O → H2 + CO2 [Water-Gas Shift Reaction]
CnHm + n H2O → n CO + (n + 1/2m) H2
Note: B-T-E’s CO2 Conversion Technology was verified “experimentally” in a 12.5 ton/day Pilot Plant.
Note: B-T-E, Inc. owns and holds the rights to the Patented B-T-E CO2 Conversion Technology with 22 Patents and Patents Pending for protecting business ventures.
B-T-E’s Technology used for producing Ethanol from Landfill Gas (LFG), i.e., LFG-Ethanol, is “new” highly profitable technology for supporting effective waste management such as environmentally “capture of more Landfill Gas from the Landfill for the public good.” Linn County, City of Cedar Rapids, CRLCSWA, City of Marion, and Business/Investors could use the positive net annual revenue from the LFG-Ethanol plant for the benefit of the environment and community.
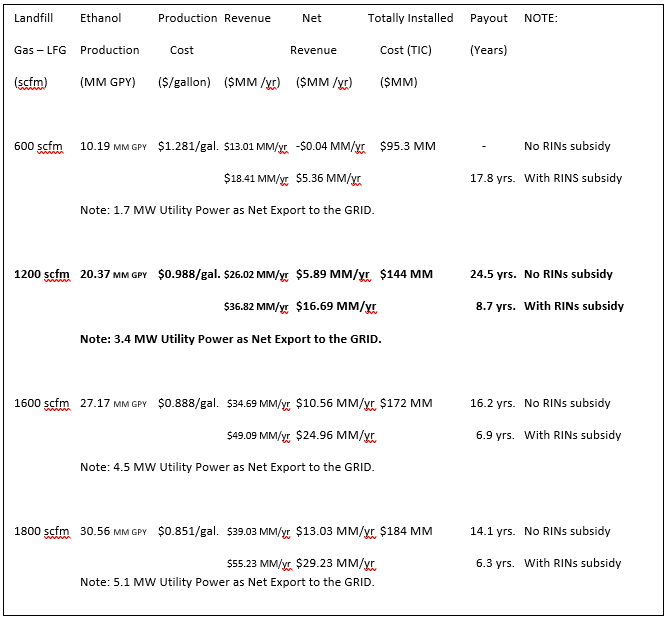
Preliminary economics was done for producing Ethanol from the Landfill Gas (LFG) at site-2, Landfill, Cedar Rapids Linn County Solids Waste Agency (CRLCSWA). Summary of Preliminary Analysis for Site-2 using Landfill Gas to Ethanol Plant at various Landfill Gas (LFG) capacities such as 600 scfm, 1200 scfm, 1600 scfm, and 1800 scfm are listed below with Ethanol selling price of $1.2770/gal., Natural gas cost at $2.733/MMBtu, Electricity at $0.0693 /kw-hr. A subsidy for RINs at $0.5330, D5 (“Other” Adv. Bio) was used where applicable.
As shown in the table below are the preliminary economics of a Landfill Gas-to-Ethanol Plant at various capacities for Landfill site-2 which can be installed. For example at a plant capacity of 600 scfm of Landfill Gas (LFG), the LFG-Ethanol plant can be profitable with the existing subsidy for Ethanol and produce 10.19 MM GPY Ethanol with a Totally Installed Plant Cost (TIC) of $95.3MM.
Landfill Gas (LFG) from Site-2 according to SCS Engineers was about 800 scfm “recovery from existing/planned system” for year-2019 and 1,200 scfm for “recovery potential.” Landfill Gas “recovery potential” is between 1,200 scfm – 1,600 scfm between year-2019 – year-2030. Landfill Gas at Site-2 should be near 1,200 scfm by the latter half of year-2019.
The 600 scfm LFG-Ethanol plant can be expanded profitably to recover more LFG for production of Ethanol. As shown, the plant can be expanded over time as needed by an incremental amount to recover 1800 scfm or more of LFG as Ethanol, profitably. Also, the LFG-Ethanol plant generates the funds for increasing the recovery of Landfill Gas (LFG) from the Landfill which increases the economy of scale for the LFG-Ethanol plant, i.e., a win-win situation.
In addition, Leachate from the Landfill can be recycled to the Landfill creating additional Landfill Gas (CO2 & CH4) for production of additional Ethanol and reducing the need to truck Leachate to a Waste Treatment Plant for processing and final disposal of solids to a landfill.
Consequently with recycle of Leachate, the following preliminary technical and economic analysis “should be exceeded” for LANDFILL Site-2 of Cedar Rapids Linn County Solid Waste Agency (CRLCSWA):
LFG Ethanol & Production Cost Revenue Net Revenue TIC Payout
1200 scfm 20.37 MM GPY $0.988/gal. $26.02 MM/yr $5.89 MM/yr $144 MM 24.5 yrs. No RINs subsidy
$36.82 MM/yr $16.69 MM/yr 8.7 yrs. With RINs subsidy
Note: 3.4 MW Utility Power as Net Export to the GRID.
As another example of the economics of a Landfill using B-T-E’s Patented CO2 Conversion Process for the conversion of Landfill Gas (LFG) to Ethanol, such as at the CRLCSWA Landfill site-2. From the table above at Landfill Gas LFG rate of 1600 scfm, Ethanol production is 27.17 MM GPY at a Production Cost of $0.888/gallon. Revenue is $34.69 MM/year and Net Revenue is $10.56 MM/year. Capital is $172 MM (TIC, Totally Installed Cost) at 6% for 20 years. Payout is 16.2 years with no subsidy considered (no RINs). With a subsidy considered, Revenue is $49.09 MM/year and Net Revenue is $24.96 MM/year with payout of 6.9 years. Note, 4.5 MW Utility Power is available as Net Export to the GRID.
A feasibility study would be the next logical step for this technology, i.e. B-T-E’s Patented CO2 Conversion Technology used to produce Ethanol from Landfill Gas (LFG). Feasibility study would be conducted by a large independent engineering firm, Bio-Thermal-Energy, Inc., Cedar Rapids Linn County Solid Waste Agency, and business/investors.
Example E: B-T-E’s Patented CO2 Technology for Conversion of Landfill Gas (LFG) into Ethanol, Current Economic, December 2020
Turning Landfill Gas (LFG) into Ethanol
Process converts LFG (carbon dioxide and methane) with water into syngas with further fermentation to Ethanol
By Gary C. Young | December, 2020


Landfill Gas (LFG) -----> to -----> Ethanol
A new process for the economic conversion of Landfill Gas (Carbon dioxide, CO2 & Methane, CH4) into ethanol has been developed, i.e., Landfill Gas (LFG)-Ethanol. The process was developed and patented by Bio-Thermal-Energy, Inc. of Cedar Rapids, Iowa specifically for improving the economic and environmental benefits for a Landfill. The new process generates a net annual revenue without a subsidy and reduces Carbon emissions from the landfill by 56.9%.
The Process
An illustration of the over-all process from Landfill Gas (LFG)-to-Ethanol is as follows:
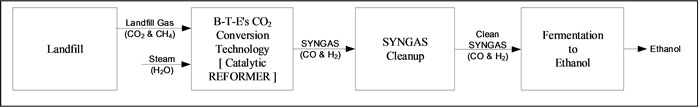
Landfill Gas (CO2 & CH4) & Steam (H2O) -to- REFORMER -to- SYNGAS (CO & H2) -to- Fermentation -to- Ethanol
As illustrated, Landfill Gas (LFG), typically 50% Methane (CH4) and 50% Carbon dioxide (CO2) with some impurities is cleaned up prior to entering a Catalytic Reformer with Steam for conversion to Syngas (CO and H2). After Syngas cleanup, the Syngas is fermented to Ethanol using an existing well-established commercial process. A Carbon balance about the entire process from Landfill Gas to Ethanol indicates that about 56.9% of the Carbon is contained in the finished product, Ethanol. Thus, 56.9% of the Carbon from the Landfill Gas is “NOT” emitted to the atmosphere and represents a Carbon emission reduction.
In addition, Leachate from the Landfill can be recycled to the Landfill creating additional Landfill Gas for production of additional Ethanol. Also, leachate recycle reduces the need to truck Leachate to a Waste Treatment Plant for processing and final disposal of solids to a landfill.
The Landfill Gas (LFG)-to-Ethanol Plant also provides funds for maintaining the landfill as required by regulatory agencies even after closing of the landfill operations. Landfill Gas is produced, typically, for more than 20 years after a landfill is closed.
Economic Analysis
The economics of the process for the conversion of Landfill Gas-to-Ethanol was estimated for a system in which the Landfill Gas (LFG) was produced at a rate of 1200 scfm (standard cubic feet per minute), and considered as waste with no cost. The total production cost for the LFG-Ethanol plant was $0.946 per gallon for the ethanol with an estimated production of 20.37 million gallons Ethanol per year and Totally Installed Cost (TIC) of $144MM. Electrical cost of $0.0500 per kWh. Totally Installed Cost (TIC) includes ISBL (inside battery limits) and OSBL (outside battery limits) with OSBL at 50 percent of ISBL, which is typical of a Greenfield plant. In the economic analysis, total CAPEX includes ISBL and OSBL, capital at 6 percent and 20 years, OPEX-operation expense includes labor and maintenance. In addition to the production of 20.37 million gallons Ethanol per year, 3.4 MW Utility Power is produced for Net Export to the GRID.
From a preliminary economic analysis, a net revenue (revenue – cost of production) of $11.65 MM / year is produced with Ethanol selling at $1.5175 / gallon and “no” subsidy considered.
Net revenue of $28.25 MM / year is generated with a subsidy. Subsidy for Ethanol is $0.815 / gallon per the Chicago Board of Trade for D5(“Other”Adv.Bio).
B-T-E’s CO2 Conversion Technology has several patents, both U.S. Patents and Foreign Patents. In addition, the technology was proven experimentally in a pilot plant.
Current business plan is to work with a Landfill owner and have a technical and economic feasibility study done by a large independent engineering firm for a specific Landfill operation to convert the Landfill Gas (LFG) into ethanol.
If the feasibility study of the Landfill demonstrates environmental benefits and profitability, the next step will be a pilot project and/or commercial project.